Here I will show how to parse PDB and PDBx/mmCIF files to obtain some structural data, and possibly to visualise the 3D conformation. We will use Biopython, which is a fairly comprehensive Python library to handle many use cases. Before starting, don’t forget to install it:
pip install biopythonFocussing on structural data, Biopython can parse most of the standard file formats (PDB, PDBx/mmCIF, and more). We start by adding some magic to remove annoying warnings (don’t do this while you are coding: some warnings are useful!)
import warnings
from Bio import BiopythonWarning
warnings.simplefilter('ignore', BiopythonWarning)
import matplotlib.pyplot as plt
import numpy as npThen we parse two files containing the 1a3n structure. Note that you should download these files and put them in the current working folder beforehand: 1a3n.pdb and 1a3n.cif.
from Bio.PDB.PDBParser import PDBParser
pdb_parser = PDBParser(PERMISSIVE=1)
pdb_structure = pdb_parser.get_structure("1a3n", "1a3n.pdb")
from Bio.PDB.MMCIFParser import MMCIFParser
cif_parser = MMCIFParser()
cif_structure = cif_parser.get_structure("1a3n", "1a3n.cif")The only difference between the two structures is the file they have been read from, so in the following we will be using only one of them.
Here I refer to Biopython’s documentation. The Structure object follows the so-called SMCRA (Structure/Model/Chain/Residue/Atom) architecture:
A structure consists of models
A model consists of chains
A chain consists of residues
A residue consists of atoms
The hierarchy can be traversed quite naturally:
i = 0
for model in cif_structure:
for chain in model:
for residue in chain:
for atom in residue:
if i < 10: # print the first ten to avoid flooding the output
print(atom)
i += 1<Atom N>
<Atom CA>
<Atom C>
<Atom O>
<Atom CB>
<Atom CG1>
<Atom CG2>
<Atom N>
<Atom CA>
<Atom C>
List of entities can also be obtained directly, and there exist some auxiliary functions that make life easier:
# Iterate over all residues
for i, residue in enumerate(cif_structure.get_residues()):
if i < 10:
print(residue, residue.get_parent())
# Iterate over all atoms
for i, atom in enumerate(cif_structure.get_atoms()):
if i < 10:
print(atom)<Residue VAL het= resseq=1 icode= > <Chain id=A>
<Residue LEU het= resseq=2 icode= > <Chain id=A>
<Residue SER het= resseq=3 icode= > <Chain id=A>
<Residue PRO het= resseq=4 icode= > <Chain id=A>
<Residue ALA het= resseq=5 icode= > <Chain id=A>
<Residue ASP het= resseq=6 icode= > <Chain id=A>
<Residue LYS het= resseq=7 icode= > <Chain id=A>
<Residue THR het= resseq=8 icode= > <Chain id=A>
<Residue ASN het= resseq=9 icode= > <Chain id=A>
<Residue VAL het= resseq=10 icode= > <Chain id=A>
<Atom N>
<Atom CA>
<Atom C>
<Atom O>
<Atom CB>
<Atom CG1>
<Atom CG2>
<Atom N>
<Atom CA>
<Atom C>
How do we extract the sequence of the chains composing the structure (or model, more correctly)? We have to first convert the structure to a list of peptides, and then we can use the get_sequence method:
from Bio.PDB.Polypeptide import PPBuilder
builder = PPBuilder()
peptides = builder.build_peptides(cif_structure[0])
for i, peptide in enumerate(peptides):
print("Chain n.", i)
print(peptide.get_sequence())Chain n. 0
VLSPADKTNVKAAWGKVGAHAGEYGAEALERMFLSFPTTKTYFPHFDLSHGSAQVKGHGKKVADALTNAVAHVDDMPNALSALSDLHAHKLRVDPVNFKLLSHCLLVTLAAHLPAEFTPAVHASLDKFLASVSTVLTSKYR
Chain n. 1
HLTPEEKSAVTALWGKVNVDEVGGEALGRLLVVYPWTQRFFESFGDLSTPDAVMGNPKVKAHGKKVLGAFSDGLAHLDNLKGTFATLSELHCDKLHVDPENFRLLGNVLVCVLAHHFGKEFTPPVQAAYQKVVAGVANALAHKYH
Chain n. 2
VLSPADKTNVKAAWGKVGAHAGEYGAEALERMFLSFPTTKTYFPHFDLSHGSAQVKGHGKKVADALTNAVAHVDDMPNALSALSDLHAHKLRVDPVNFKLLSHCLLVTLAAHLPAEFTPAVHASLDKFLASVSTVLTSKYR
Chain n. 3
HLTPEEKSAVTALWGKVNVDEVGGEALGRLLVVYPWTQRFFESFGDLSTPDAVMGNPKVKAHGKKVLGAFSDGLAHLDNLKGTFATLSELHCDKLHVDPENFRLLGNVLVCVLAHHFGKEFTPPVQAAYQKVVAGVANALAHKYH
Note that we can also directly extract some interesting information. We can write a function that computes the gyration radius of the atoms:
def gyration_radius(peptide):
com = np.zeros(3, dtype="float")
N_residues = 0
for residue in peptide:
if "CA" in residue:
com += residue["CA"].get_coord()
N_residues += 1
com /= N_residues
Rg2 = 0
for residue in peptide:
if "CA" in residue:
diff = com - residue["CA"].get_coord()
Rg2 += np.dot(diff, diff)
Rg2 /= N_residues
return np.sqrt(Rg2)
for i, peptide in enumerate(peptides):
print("Chain", i, "- Radius of gyration:", gyration_radius(peptide))Chain 0 - Radius of gyration: 14.350959669272358
Chain 1 - Radius of gyration: 14.82890768992068
Chain 2 - Radius of gyration: 14.340855886355154
Chain 3 - Radius of gyration: 14.884173860393785
We can also compute the dihedral angles of the chain backbones:
phis = []
psis = []
for peptide in peptides:
angles = peptide.get_phi_psi_list()
phis += [angle[0] for angle in angles if angle[0] is not None]
psis += [angle[1] for angle in angles if angle[1] is not None]
plt.hist(phis, label="$\phi$", alpha=1)
plt.hist(psis, label="$\psi$", alpha=0.2)
plt.xlabel("Angle")
plt.ylabel("Counts")
plt.legend()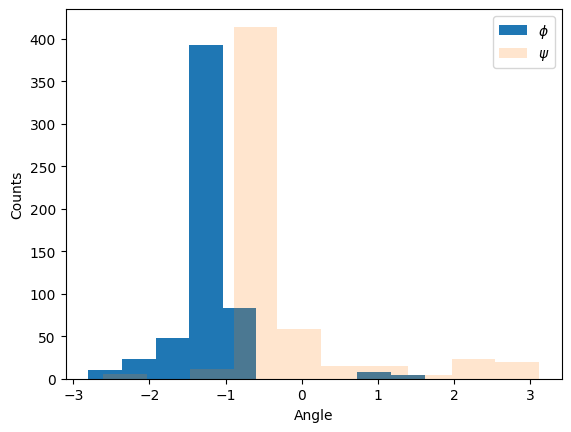
If we want to compute something a bit less standard (like the dihedral, which is the rotation around the peptide bond), we have to write the logic ourselves: here we pass the positions of the and atoms of residue and of the and atoms of residue to the Biopython’s calc_dihedral function, which computes and returns the associated dihedral, which in this case is .
from Bio.PDB.vectors import calc_dihedral
omegas = []
for peptide in peptides:
for res in range(0, len(peptide) - 1):
# get vectors for this peptide and the N from the subsequent peptide
CA = peptide[res]['CA'].get_vector()
C = peptide[res]['C'].get_vector()
N_next = peptide[res + 1]['N'].get_vector()
C_next = peptide[res + 1]['CA'].get_vector()
omega = calc_dihedral(CA, C, N_next, C_next)
omegas.append(abs(omega))
plt.hist(phis, label="$\phi$", alpha=1)
plt.hist(psis, label="$\psi$", alpha=0.2)
plt.hist(omegas, label="$\omega$", alpha=1)
plt.xlabel("Angle")
plt.ylabel("Counts")
plt.legend()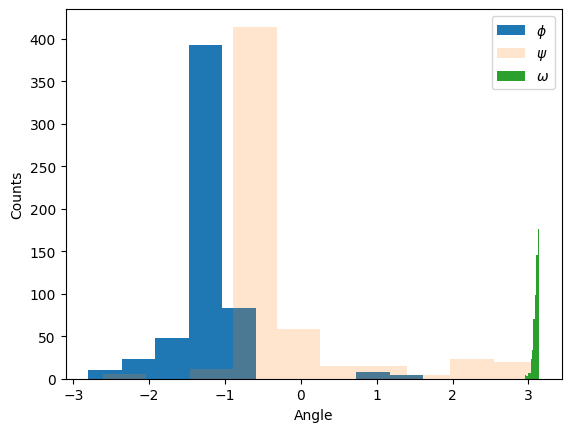
1Visualisation¶
In addition to the tools discussed during the lecture, it is also possible to visualise a PDB file directly in a Jupyter notebook with nglview, which can be installed on conda with:
$ conda install nglview -c conda-forgeor through pip:
$ pip install nglviewNota Bene: in some cases you also have to manually install jupyterlab, and often the visualisation will not work regardless. Using a different browser can help
import nglview
print(nglview.__version__)4.0
view = nglview.show_file("1a3n.pdb")view = nglview.show_biopython(pdb_structure)
view2The gyration radius as a function of protein size¶
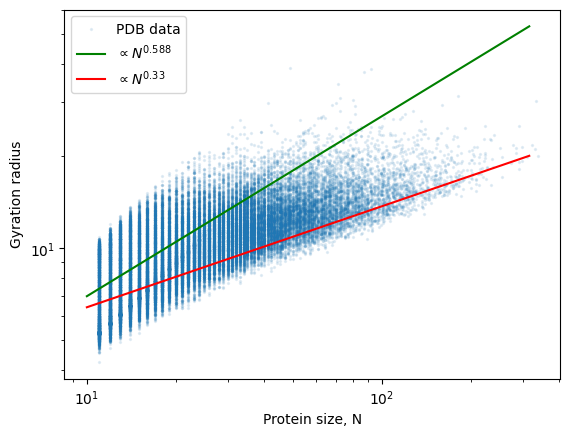
We can compute the gyration radius of many proteins to see how depends on the number of residues . Note that to run the next cell you will have to download a file containing a curated list of PDB files from here. Be careful, the smallest file (https://zenodo.org/records/5777651/files/top2018_pdbs_mc_filtered_hom30.tar.gz?download=1) is almost 1 GB in size! Then you will have to unpack the archive file and update the path in the snippet below.
Note that the analysis will take at least several minutes, depending on your computer.
from glob import glob
sizes = []
radii = []
for i, pdb in enumerate(glob("/home/lorenzo/Downloads/top2018_pdbs_mc_filtered_hom30/*/*/*pdb")):
structure = pdb_parser.get_structure(pdb[:-4], pdb)
peptides = builder.build_peptides(structure[0])
for peptide in peptides:
if len(peptide) > 10:
sizes.append(len(peptide))
radii.append(gyration_radius(peptide))If we plot the results, we can see that small proteins behave more or less like self-avoiding walks, while large ones appear more compact, as their gyration radius seems to scale with an exponent that is closer to rather than 0.588.
plt.scatter(sizes, radii, s=2, alpha=0.1, label="PDB data")
x = np.logspace(1, 2.5)
y = 1.8 * x**0.588
plt.plot(x, y, 'g', label="$\propto N^{0.588}$")
y = 3 * x**0.33
plt.plot(x, y, 'r', label="$\propto N^{0.33}$")
plt.xscale("log")
plt.yscale("log")
plt.xlabel("Protein size, N")
plt.ylabel("Gyration radius")
plt.legend()